WELCOME TO AMIVAS
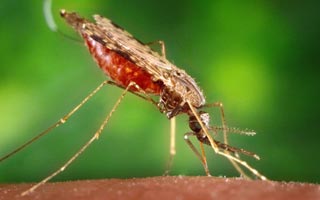
We are a pharmaceutical company delivering life-saving medicines to the world. We develop, commercialize and distribute Artesunate for severe malaria.
Therapeutics to fight life-threatening diseases
AMIVAS is a US, Australian, and European joint venture focused on the development and commercialization of therapeutics for the treatment of life-threatening diseases with offices in the US, Australia and Ireland. AMIVAS manufactures in North America and our partners in Canada, USA and Europe package and supply our product to hospitals and patients in need.
AMIVAS’ vision was formed in response to the urgent need for a US and Europe- based firm to assume responsibility for the manufacture and distribution of Artesunate, for the treatment of severe malaria after quinidine gluconate was discontinued in 2019. With the US Food and Drug Administration (FDA) approval of its first commercial product on May 26, 2020, AMIVAS is proud to join the global effort to fight and eradicate malaria.
OUR MISSION
To be the global leader in the battle against infectious diseases, driving scientific discovery and breakthroughs that will redefine the possibilities of critical medicines.
OUR VISION
To be the global leader in the development and supply of life-saving critical medicines worldwide.
Information for health professionals
Artesunate for the treatment of severe malaria. Fast, effective & easy to use.
Order Artesunate
How to order Artesunate in your country – ordering instructions, links & phone numbers.
Artesunate for Injection® – USA Artesunate Amivas® – EU